Ahmed, Shawn
Our research group has interests in telomere biology and germline immortality. We are studying these problems using the nematode Caenorabditis elegans, a multicellular eukaryote with excellent genetics, which can be combined with biochemical and cell biology approaches.
How does the germline achieve immortality?
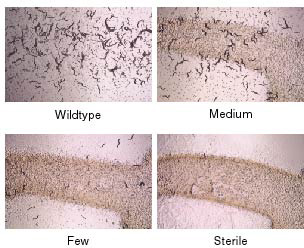
The germline is an immortal cell lineage that is passed from one generation to the next, indefinitely. In order to determine how germ cells achieve immortality, we have identified C. elegans mutants with Mortal Germlines: mutants that reproduce normally for several generations, but eventually became sterile (Figure 1). Gametes of these mutants transmit forms of stress that can accumulate over the generations to levels that cause strong effects on germ cell development (sterility or reproductive arrest). Stress transmitted by mortal germline mutants could also affect somatic development. One pathway that promotes germ cell immortality is the Piwi/piRNA genome silencing pathway. Deficiency for this pathway allows for growth for many generations but then results in an adult reproductive arrest phenotype that resembles a response to acute starvation. Furthermore, deficiency for the daf-2 insulin/IGF1 receptor, which results in stress resistance and adult longevity, can suppress the sterility of Piwi mutants. This creates an interesting link between germ cell immortality and somatic longevity whose basis we seek to understand.
Given that the soma is only needed for a single generation, evolutionary theory posits that somatic cells may be deficient for pathways that ensure germ cell immortality. Substantial evidence from vertebrates indicates that this is likely to be the case. We utilize genetic, cell biology and biochemical approaches to study pathways that enable germ cells to achieve proliferative immortality in C. elegans. This work could shed light on epigenetic forms of macromolecular stress that can be transmitted by gametes and could be generally relevant to heredity and possibly to human disease.
Analysis of Telomere Biology
A second pathway that promotes germ cell immortality is telomerase, which is a ribonucleoprotein that adds repeats to chromosome termini. The ends of linear chromosomes, telomeres, are usually composed of simple repetitive sequences whose length is maintained by telomerase. Humans deficient for telomerase suffer from the lethal hereditary disorders Aplastic Anemia, Dyskeritosis Congenita and Pulmonary Fibrosis. C. elegans is the most highly evolved multicellular eukaryote in which an unbiased genetic approach can be used to study the problem of telomere replication. A fraction of the mortal germline mutants described above are completely defective for telomerase activity in vivo (Figure 2). Studies of these genes may help to elucidate the mechanism by which telomerase functions at chromosome termini and may provide significant insight into telomerase-related diseases. We also study proteins that bind to telomeres, some of which form nuclear foci that are dynamic during development.
Telomerase is deficient in most human somatic cells, and telomere erosion in this context may contribute to genome instability that fuels the development of many forms of cancer. C. elegans has holocentric chromosomes, so fused chromosomes that occur when telomerase is deficient can be genetically isolated and mapped. These stable end-to-end chromosome fusions allow us to address the mechanism by which dysfunctional telomeres are repaired with unparalleled clarity, and may provide insight into how large-scale DNA rearrangements occur during tumorigenesis.