
Duronio, Robert J.
Professor & Department Chair
Director of the Integrative Program for Biological and Genome Sciences
Affiliations: Department of Genetics; Curriculum in Genetics & Molecular Biology; Director, Integrative Program for Biological and Genome Sciences (iBGS)
duronio@med.unc.edu
3350 Genome Sciences Building
(919) 962-4568 (office)
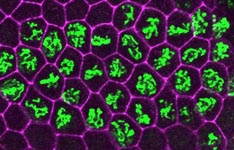
At a Glance
- Epigenetic control of genome structure and function
- Cell cycle-regulated gene expression
- Developmental genetics
Synopsis
Our research focuses on understanding the molecular mechanisms that regulate DNA replication and cell proliferation during animal development. An orderly process of events called the cell cycle, which in its most familiar form consists of four phases (G1-S-G2-M) controls cell proliferation. The genome is replicated during the “S” or synthesis phase and duplicated chromosomes are segregated to daughter cells during the “M” or mitotic phase when cell division occurs. G1 and G2 are “gap” phases during cells regulate the entry into S phase and M phase, respectively. We study gene expression events that control how cells make the decision to enter S phase and proliferate, or to exit the cell cycle and differentiate. Without such control there would be no coordination between cell proliferation and the development and function of the many different types of tissues that make up an organism. In addition, the breakdown of cell cycle regulation is one of the events that contribute to the generation of cancer.
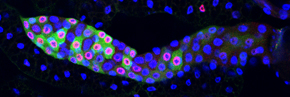
We study this problem using the fruit fly Drosophila melanogaster, in part because the genes controlling cell proliferation in fruit flies have been highly conserved during evolution and function the same way as in other animals, including humans. This allows us to exploit certain advantages that Drosophila has as a research tool, including the ease with which genetics (making and analyzing mutants) and cell biology (using microscopy to
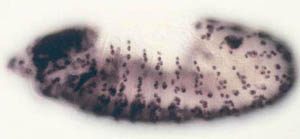
observe cell proliferation) can be applied to the study of gene function in the context of a whole animal. Some of the cell cycle regulatory pathways that we study become defective in virtually every human cancer. Thus, one hope is that understanding how these pathways function in normal Drosophila development will give us clues to how they might malfunction in the deregulated growth typical of cancer.